41 label each reactant and product in the given chemical reaction
Label each reactant and product in the given chemical reaction.CH4+ 2O2 ... Label each reactant and product in the given chemical reaction.CH4+ 2O2 CO2 +2H2O Expert's Answer Solution.pdf Next Previous Q: Q: View Answer Q: View Answer Q: (a) Methane, CH4, burns in the presence of oxygen to form water vapor and carbon dioxide. Write a balanced chemical equation for this combustion reaction. Chemical Reactions Calculator - Symbolab Examples. One Time Payment $12.99 USD for 2 months. Weekly Subscription $2.49 USD per week until cancelled. Monthly Subscription $6.99 USD per month until cancelled. Annual Subscription $29.99 USD per year until cancelled.
chem.libretexts.org › Bookshelves › General_Chemistry15.2: The Equilibrium Constant (K) - Chemistry LibreTexts Aug 14, 2020 · Given: balanced equilibrium equation, \(K\) at a given temperature, and equations of related reactions. Asked for: values of \(K\) for related reactions. Strategy: Write the equilibrium constant expression for the given reaction and for each related reaction. From these expressions, calculate \(K\) for each reaction. Solution:

Label each reactant and product in the given chemical reaction
courses.lumenlearning.com › suny-mcc7.3 Classifying Chemical Reactions | Introductory Chemistry 26. Use the solubility rules to predict if each double-replacement reaction will occur and, if so, write a balanced chemical equation. a. KC 2 H 3 O 2 + Li 2 CO 3 → ? b. KOH + AgNO 3 → ? 27. Use the solubility rules to predict if each double-replacement reaction will occur and, if so, write a balanced chemical equation. a. K 3 PO 4 + SrCl 2 ... Chemical equations - Types of reaction - KS3 Chemistry Revision - BBC ... Here is the balanced symbol equation: 2Cu + O2 → 2CuO. You can see that we now have two copper atoms and two oxygen atoms on each side. This matches what happens in the reaction: Two copper ... Reactants and Products | Chemistry for Non-Majors | | Course Hero A reactant is a substance that is present at the start of a chemical reaction. The substance (s) to the right of the arrow are called products . A product is a substance that is present at the end of a chemical reaction. In the equation above, the zinc and sulfur are the reactants that chemically combine to form the zinc sulfide product.
Label each reactant and product in the given chemical reaction. Worked example: Calculating amounts of reactants and products A balanced chemical equation shows us the numerical relationships between each of the species involved in the chemical change. Using these numerical relationships (called mole ratios), we can convert between amounts of reactants and products for a given chemical reaction. Solved 1. Label each reactant and product in the given | Chegg.com See the answer 1. Label each reactant and product in the given chemical reaction. CH4 + 2O2 CO2+ 2H2OCH4 + 2O2 CO2 + 2H2O Answer Bank: Product/Reactant 2. Use the law of conservation of mass to answer the questions. Consider a hypothetical reaction in which A and B are reactants and C and D are products. chem.libretexts.org › Bookshelves › General_Chemistry14.3: Effect of Concentration on Reaction Rates: The Rate Law Feb 03, 2022 · For each reaction, give the units of the rate constant, give the reaction order with respect to each reactant, give the overall reaction order, and predict what happens to the reaction rate when the concentration of the first species in each chemical equation is doubled. \(\mathrm{2HI(g)}\xrightarrow{\textrm{Pt}}\mathrm{H_2(g)}+\mathrm{I_2(g)} Reaction profiles - Exothermic and endothermic reactions - AQA - BBC It shows the energy in the reactants and products, and the difference in energy between them. Exothermic reaction The energy level decreases in an exothermic reaction. This is because energy is...
Label The Following Reaction Coordinate Diagram. - Chapter 1 ... Label The Following Reaction Coordinate Diagram. - Chapter 1 Introduction And Overview Of Electrogenerated Chemiluminescence Rsc Publishing Doi 10 1039 9781788015776 00001 ... Reactants and Products - GeeksforGeeks The reactants in the formation of water are hydrogen (H2) and oxygen (O 2) gas. Water is the product (H 2 O). Carbon dioxide (CO 2) and water are the reactants in photosynthesis (H 2 O). Glucose is the product (C 6 H 12 O 6 ). It should be noted that sunlight is not a reactant. Reactants are matter (atoms, molecules, and ions) rather than energy. How would you label each formula in the chemical equation below as ... Explanation: The reactants are on the LEFT HAND SIDE of the equation. The products are on the right hand side. So for the oxidation of iron, F e +S → F eS. Iron and sulfur are the reactants, and iron sulfide is the product. Label each reactant and product in the given chemical reaction. Label each reactant and product in the given chemical reaction. Chemical reaction: A chemical reaction is a process in which two or more elements or compounds (the reactants) react together to form...
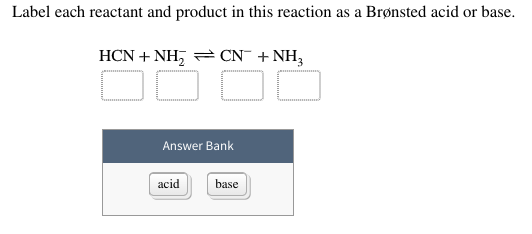
7.2 Reactants and products | Chemical reactions | Siyavula During a chemical reaction, the reactants are used to make the products. The atoms in the reactants have been rearranged into new compounds (the products). A simulation about reactants, products and leftovers A chemical reaction is a rearrangement of atoms Answered: 1 Label each reactant and product in… | bartleby Solution for 1 Label each reactant and product in this reaction as a Brønsted acid or base. HCN+NH2−↽−−⇀CN−+NH3 menu ... Well-balanced chemical reaction between phosphoric acid and liquid ammonia: ... Given reaction , Cyclopentyloxirane ... 2.4 Flashcards | Quizlet Label the reactants and products in the chemical reaction: CH4+2O2----------------------> CO2+2H2O. Write brief definitions for these terms next to their labels. CH4+2O2- reactants: the substances changed during a chemical reaction; they are located on the left side of the equation. CO2+2H2O- products: the substances made by a chemical reaction; alpha.chem.umb.edu › chemistry › ch118IODINE CLOCK REACTION KINETICS As this reaction proceeds, the colorless reactants gradually develop a brown color due to the product I2. Because of the difficulty of timing the appearance of the I2, we make use of another much faster reaction in the same solution to mark the progress of the slow reaction: I2 + 2 S2O3 2-2 I -+ S 4O6 2-fast (2) The reaction
Reactants And Products In Chemical Reactions - BYJUS Law of conservation of mass governs the balancing of a chemical equation. According to this law, mass can neither be created nor be destroyed in a chemical reaction and obeying this law total mass of the elements or molecules present on the reactant side should be equal to the total mass of elements or molecules present on the product side. If the number of atoms of the elements/molecules on the reactant side are not equal to the number of atoms of the elements/molecules on the product side ...
4.1 Writing and Balancing Chemical Equations - Chemistry The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. This is a requirement the equation must satisfy to be consistent with the law of conservation of matter.
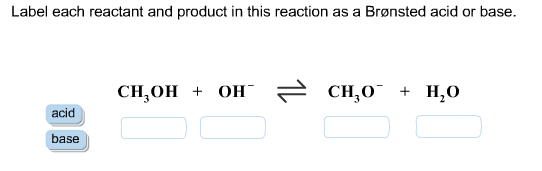
SOLVED:Label each reactant as a Brønsted-Lowry acid or a Brønsted-Lowry ... Now with bronze did Lowry acid based definitions. Then we end up creating a conjugate acid and conjugate base. On the product side, the acid produces a conjugal base and the base produces a conjugate acid. In the next chemical reaction, we see that C H three n h two is accepting the hydrogen ion.
› article › academics-the-artsWhat Factors Affect the Speed of Chemical Reactions? - dummies Mar 26, 2016 · If you want to produce as much of a product as possible as fast as possible in a chemical reaction, you must consider the kinetics of the reaction. Nature of chemical reactants. In order for a reaction to occur, there must be a collision between the reactants at the reactive site of the molecule. The larger and more complex the reactant ...
3.2 Identifying Reactants, Products, Coefficients, and ... - Quizlet Start studying 3.2 Identifying Reactants, Products, Coefficients, and Subscripts in Chemical Equations (AL). Learn vocabulary, terms, and more with flashcards, games, and other study tools. Search
) In the reaction below, label each reactant as a nucleophile or an ... Answer to ) In the reaction below, label each reactant as a nucleophile or an electrophile. CH3COO-+O2S(OCH3)2CH3COOCH3+CH3SO4-
› read › science8.3 Le Chatelier's principle | Chemical equilibrium | Siyavula The forward reaction is favoured by higher pressures because there are \(\text{2}\) gas molecules of product for every \(\text{4}\) gas molecules of reactant. Refer to Chapter 14 for more information on the Haber process and other industrial applications.
study.com › learn › chemical-reactions-questions-andChemical Reactions Questions and Answers | Study.com Consider the following reaction: 4 NaCl + 2 SO_2 + 2 H_2O + O_2 to 2Na_2SO_4 + 4 HCl For the initial amounts of each reactant as given below, choose the compound that is the limiting reactant. a) N...





Post a Comment for "41 label each reactant and product in the given chemical reaction"