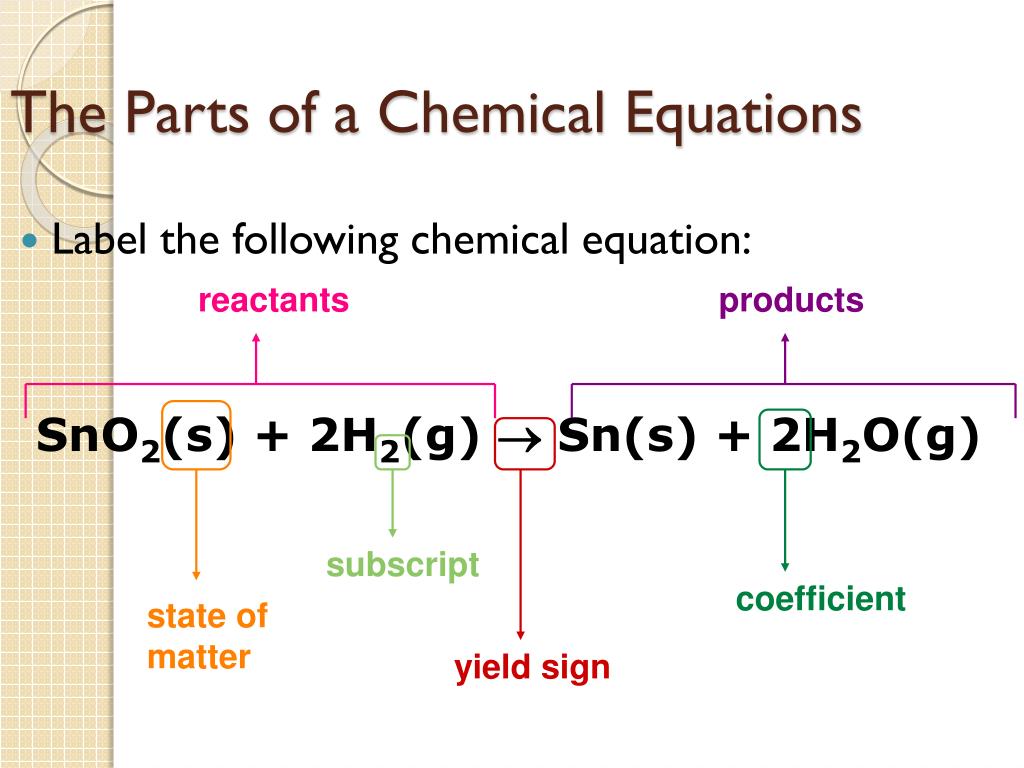
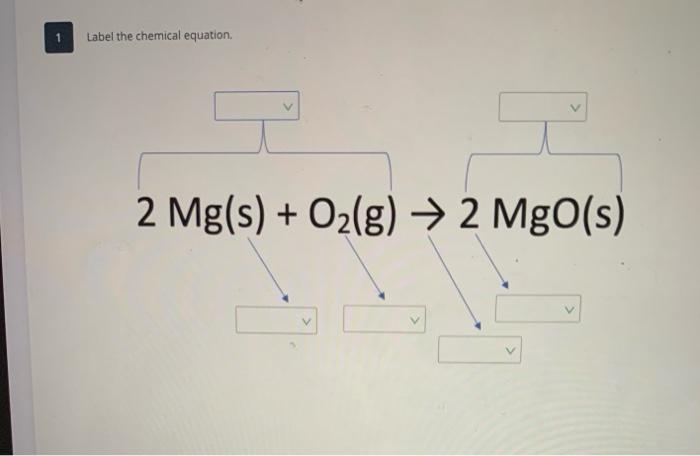
44 labeling a chemical equation
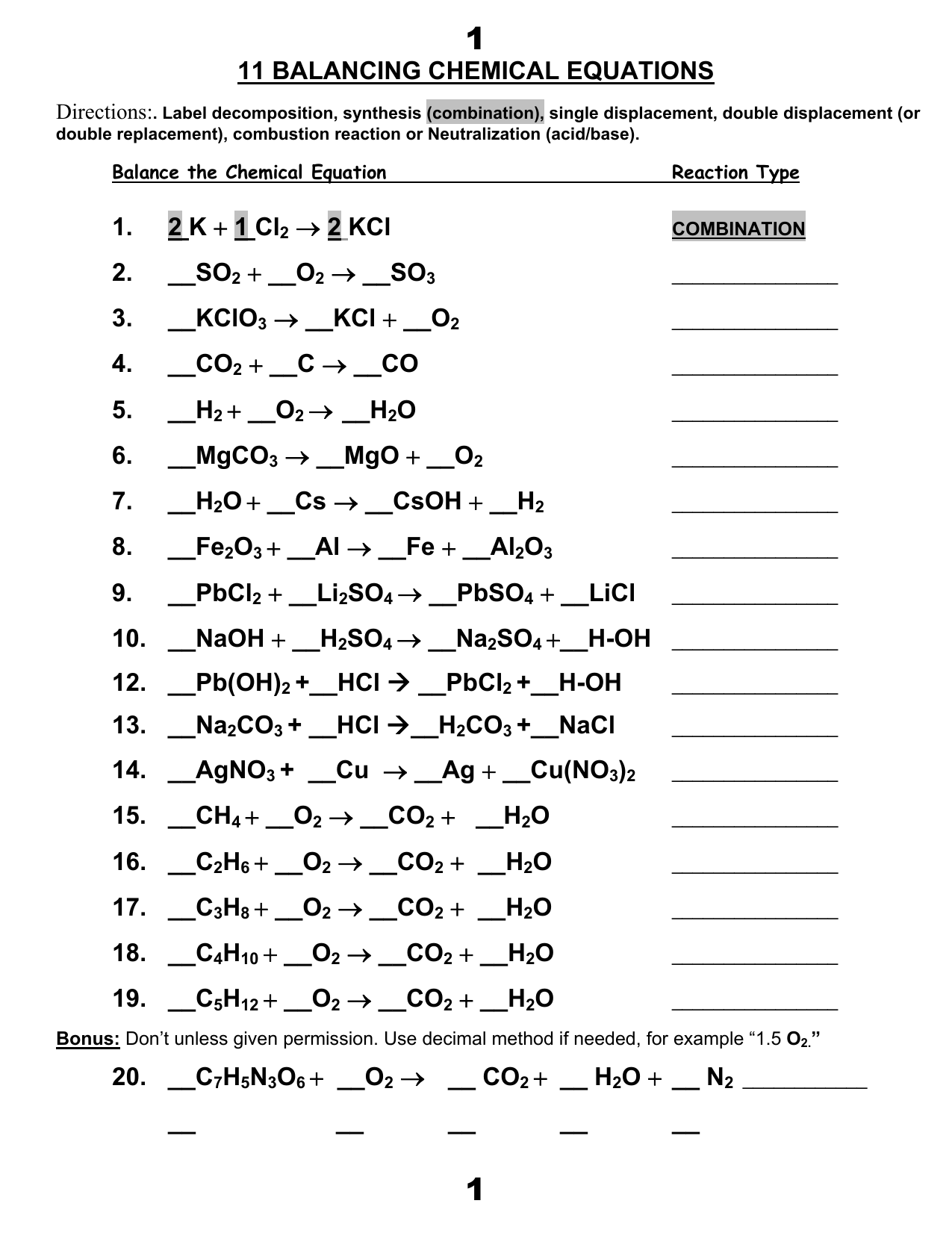
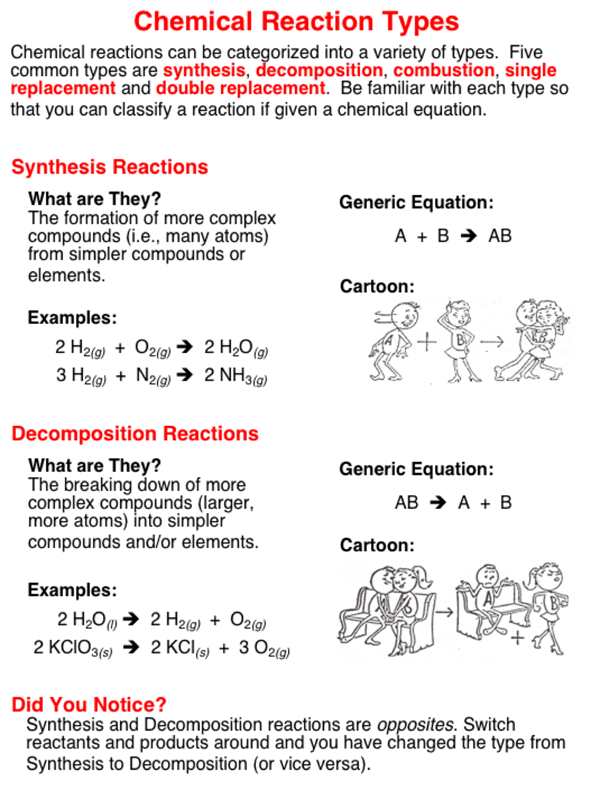
Balancing chemical equations - How to Balance Chemical Equations Easily ... c = 6a = 6*1 = 6. d = 6a = 6. Substituting the values of a,c, and d in the equation 6a + 2b = 2c + d, the value of 'b' can be obtained as follows: 6*1 + 2b = 2*6 + 6. 2b = 12; b = 6. It is important to note that these equations must be solved in a manner that each variable is a positive integer. PDF Laboratory Safety Labeling and Transfer Facts of Chemicals Facts Laboratory Safety Labeling and Transfer of Chemicals Permanent Container Labels . Employers must ensure that no worker uses, stores, or . allows any other person to use or store any hazardous . substance in a laboratory if the container (including . bags, barrels, bottles, boxes, cans, cylinders, drums
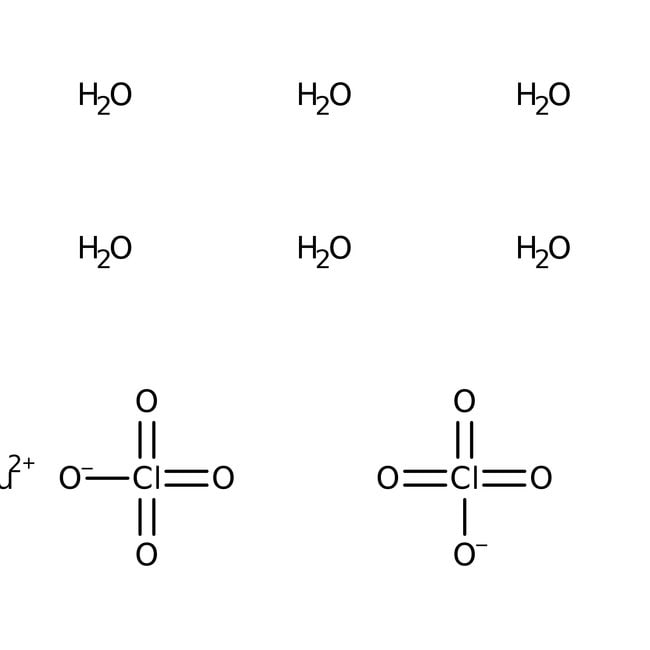
What are Chemical Equations? Detailed Explanation, Examples - BYJUS Chemical Equation: CaCl 2 + 2AgNO 3 → Ca(NO 3) 2 + 2AgCl↓. Ionic Equation: Ca 2+ + 2Cl - + 2Ag + + 2NO 3 - → Ca 2+ + 2NO 3 - + 2AgCl↓. Comparing the reactants and the products of the ionic equation and the chemical equation, it can be observed that the Ca 2+ (calcium ion) and the NO 3 - (nitrate) ions are present on both sides of the ionic equation. These ions are referred to as spectator ions because they do not participate in the chemical reaction.
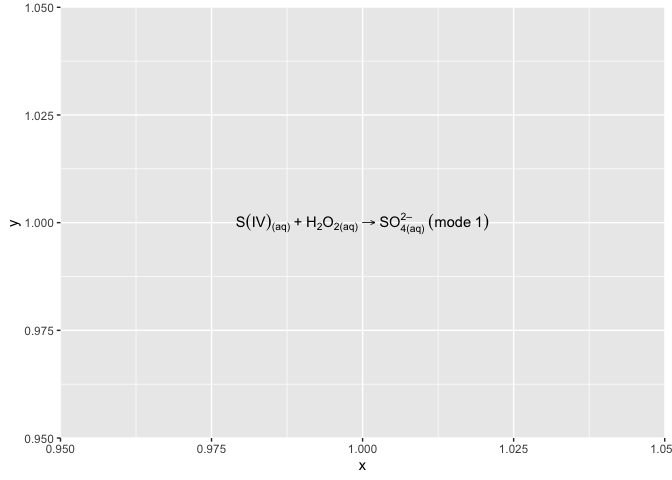
Labeling a chemical equation
The Balanced Chemical Equation for Photosynthesis - ThoughtCo Explanation. In words, the equation may be stated as: Six carbon dioxide molecules and six water molecules react to produce one glucose molecule and six oxygen molecules . The reaction requires energy in the form of light to overcome the activation energy needed for the reaction to proceed. Carbon dioxide and water don't spontaneously convert into glucose and oxygen . Classification, labelling and packaging of chemicals Classify, label and package chemical substances in line with the new CLP system and inform your users of the classification. ... The unique formula identifier (or UFI) was developed to assist poison centres with the fast and correct identification of products during emergency calls. All products containing hazardous substances must display the ... Labeling of chemical containers - periodni.com Fine-adjust the print position: Shift from left: mm. Shift from top: mm. How to print labels? In your browser click the Page Setup in the File menu (hit ALT-F if menu isn't visible). Set the Orientation to portrait and the Scale to 100%. Un-check the "Shrink to fit page width" box and check the "Print background (colors and images)".
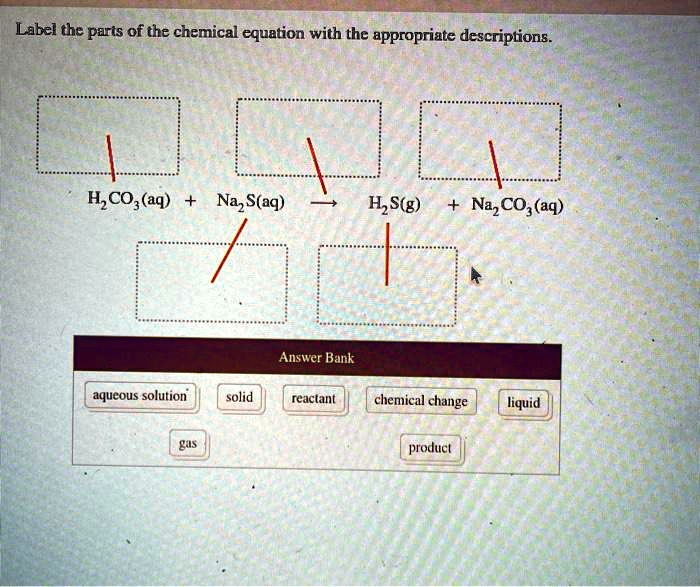
Labeling a chemical equation. What Are Chemical Equations? - ThoughtCo 2 H 2 (g) + O 2 (g) → 2 H 2 O (l) Hydrogen and oxygen are indicated by (g), which means they are gases. Water is marked (l), which means it is a liquid. Another symbol you may see is (aq), which means the chemical species is in water — or an aqueous solution. Label the chemical Equation worksheet - liveworksheets.com Label the chemical Equation Label the parts of the chemical Equation ID: 1570824 Language: English School subject: Science Grade/level: 5 Age: 7-9 Main content: Chemical Equation Other contents: NA Add to my workbooks (0) Download file pdf Embed in my website or blog Chemical Equation Balancer To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. The balanced equation will appear above. Use uppercase for the first character in the element and lowercase for the second character. Examples: Fe, Au, Co, Br, C, O, N, F. Ionic charges are not yet supported and will be ignored. How to Write a Chemical Equation (with Pictures) - wikiHow If you want to write a chemical equation, start by writing the chemical formulas of each reactant. Use the prefixes, such as mono-, di-, tri-, and tetra-, to figure out the number of atoms present for each element, and write this number as a subscript for each element. For example, dihydrogen monoxide would be more easily written as H2O.
The Dos and Donts of Chemical Labeling | Lab Manager We suggest the best approach to proper chemical container labeling is to list these four items on the label. Chemical Name — Spell out the name correctly and completely. Avoid using abbreviations or chemical formulas. Concentration — If the chemical is in solution, indicate the solution's molarity or strength. How can the chemical hurt you? How to Label Equations in Word: 10 Steps (with Pictures ... - wikiHow Steps 1 Open Microsoft Word. It's in the Windows menu (Windows) or in the Applications folder (macOS). 2 Click the Insert tab. It's at the top of the screen (to the right of the Home tab). 3 Click the arrow next to the "Equation" button. It's near the top-right corner of the screen. The arrow is pointing downward. A list of equations will appear. 4 Balancing Chemical Equations - PhET Balancing Chemical Equations - PhET How do you Write a Chemical Equation? - A Plus Topper Rules for writing chemical equation: Certain rules have to be followed while writing a chemical equation. The reactants taking part in the reaction are written in terms of their symbols or molecular formulae on the left-hand side of the equation. A plus (+) sign is added between the formulae of the reactants.
How to Number or Label Equations in Microsoft Word - How-To Geek Click "New Label." In the New Label window, type your left parenthesis and hit "OK." If you want to select a different number format, click "Numbering," choose what you'd like to use, and click "OK." You'll see the starting parenthesis with the first number per the formatting that you selected. Type a space, and then your closing parenthesis. Labeling A Chemical Reaction Teaching Resources | TpT - TeachersPayTeachers Topics covered include: Balancing equations, calculating numbers of atoms of a compound with coefficients, labeling parts of and symbols in a chemical equation, identifying 5 basic types of reactions, and writing balanced equations from word equations. Task cards are great for station work, for early finishers, or for extra practice. Labeling A Chemical Equation Part 2 - YouTube The instructions for labeling a chemical equation. How to include and reference equations - Overleaf In LaTeX we can label equations for easy reference within the article. Here we see how to create an equation using the \begin {equation} and \end {equation} commands. This equation is automatically numbered, and by including a \label command, we can refer to this number from anywhere within the rest of the document using the \ref command. Basic LaTeX 12: Including and referencing equations in LaTeX.
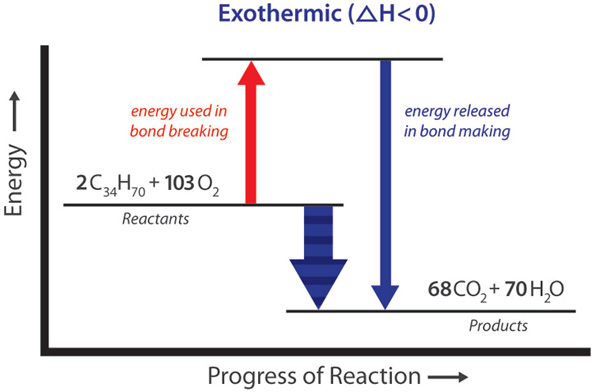
Biochemistry Equations Flashcards | Quizlet Energy interactions are greatest between integral charges and less between partial charges. U = (kq1q2)/Er (3) 1. Delta G is change in Gibbs Free Energy (spontaneous reactions always have a GFE lower than 0) 2. Delta H is change in enthalpy or heat. 3. T is the temperature (always less then 0) 4.
How to Write Chemical Equations - YouTube Now, in order to write proper chemical equations we need to know a few symbols. "g" is for gas "l" is for liquid "s" stands for solid and "aq" is for aqueous Aqueous just means dissolved in water....
Labeling of chemical containers - periodni.com Fine-adjust the print position: Shift from left: mm. Shift from top: mm. How to print labels? In your browser click the Page Setup in the File menu (hit ALT-F if menu isn't visible). Set the Orientation to portrait and the Scale to 100%. Un-check the "Shrink to fit page width" box and check the "Print background (colors and images)".
Classification, labelling and packaging of chemicals Classify, label and package chemical substances in line with the new CLP system and inform your users of the classification. ... The unique formula identifier (or UFI) was developed to assist poison centres with the fast and correct identification of products during emergency calls. All products containing hazardous substances must display the ...
The Balanced Chemical Equation for Photosynthesis - ThoughtCo Explanation. In words, the equation may be stated as: Six carbon dioxide molecules and six water molecules react to produce one glucose molecule and six oxygen molecules . The reaction requires energy in the form of light to overcome the activation energy needed for the reaction to proceed. Carbon dioxide and water don't spontaneously convert into glucose and oxygen .

























Post a Comment for "44 labeling a chemical equation"