38 phase diagram of pure solvent and solution
Binary Solid-Liquid Phase Diagram | Chem Lab For more information on binary solid-liquid phase diagrams and the derivation of these equations, please see the references. 1-4 . From your data, generate the solid-liquid phase diagram for your system and determine Δ fus H and the melting point for the two pure substances at 95% confidence. Determine the eutectic temperature and composition ... SOLVED:What are the most important differences between the phase ... You'd so, uh, you answer the first part of the question. And ah, qu'est ce solution can be defined a solution where water acts as a solvent. So what Water is our main component than it's an obvious solution. And the second part were asked about the difference between Salyut and solvent. And so, in a solution, the solvent, his our major component.
PDF Phase Diagrams Phase Diagrams Revised: 1/27/16 7 8. Slowly open the needle valve. Once the pressure within the system has equalized with atmospheric pressure, turn off the vacuum pump. 9. Melt the tert-butanol completely by suspending the 50 mL filter flask in a warm water bath. 10.
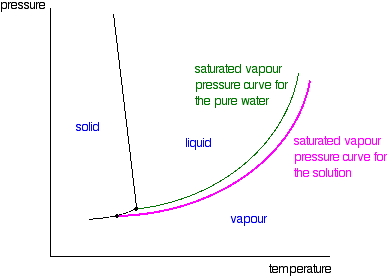
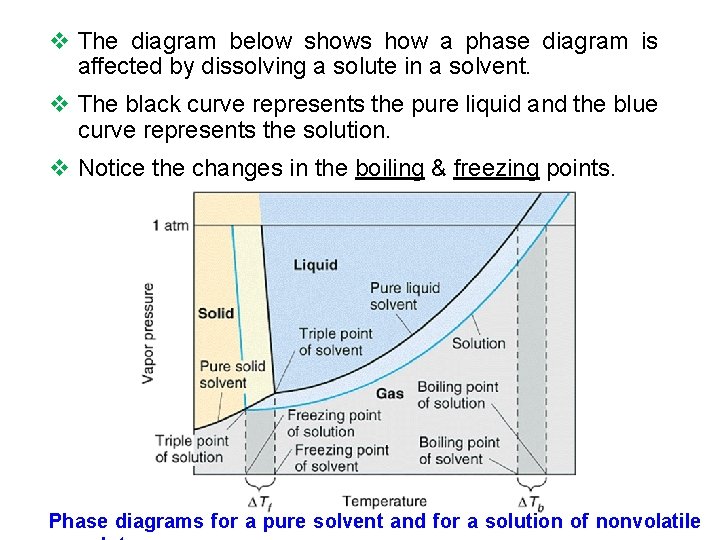
Phase diagram of pure solvent and solution
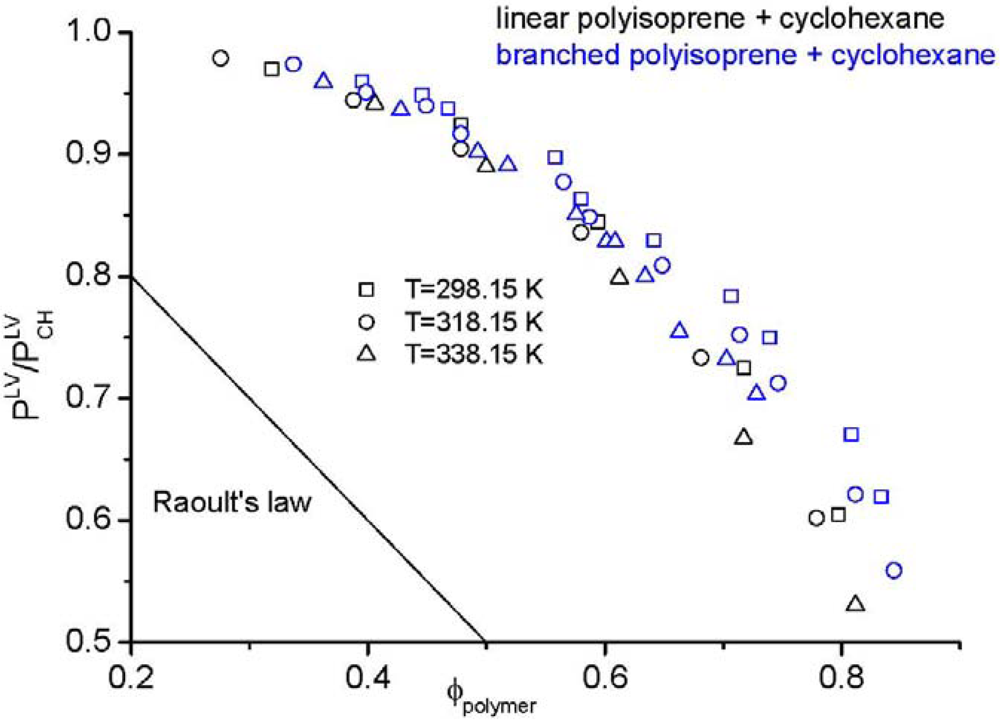
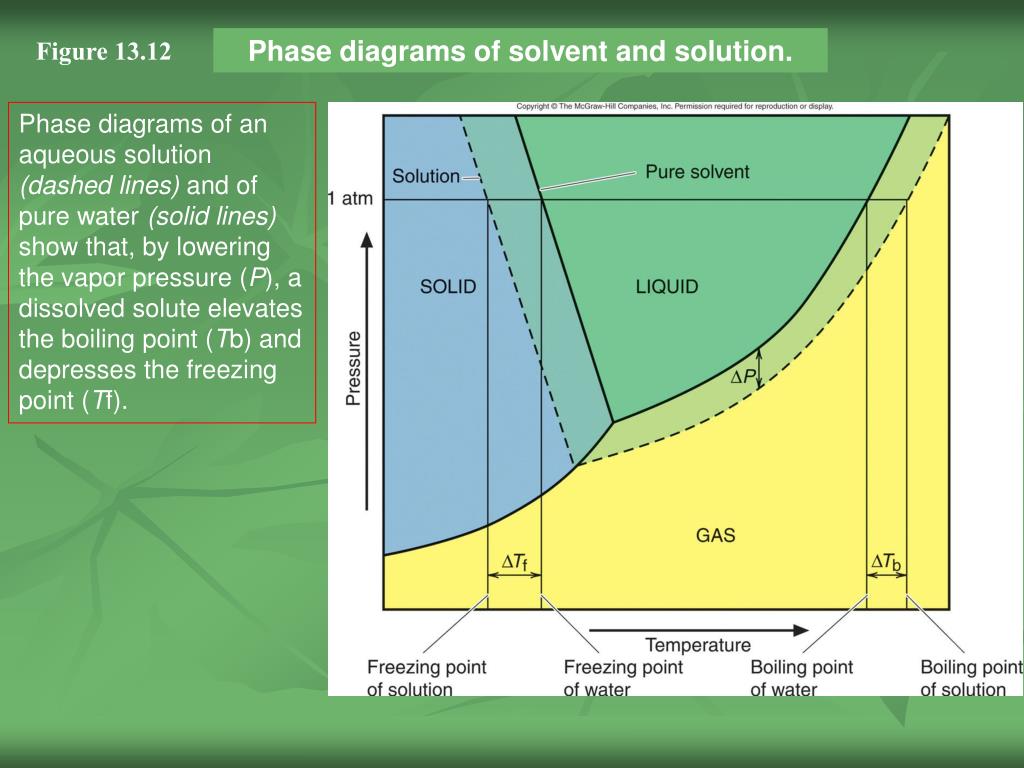
› topics › chemistryGreen Solvent - an overview | ScienceDirect Topics 4.1.1 NaOH/urea aqueous solution. As a novel green solvent, NaOH/urea aqueous solution was first used for the rapid dissolution of cellulose at low temperature by Zhang et al. [1]. Due to its excellent performance for the dissolution of biomacromolecules, Hu et al. first used NaOH/urea aqueous solution for the dissolution of chitin [2]. Process design and phase diagrams : Phasediagram Phase diagrams for pure compounds are visual tools to display the properties of pure compounds forming various phases at different temperatures and pressures. In a phase diagram for a pure component, solid-liquid, vapor-liquid, solid-vapor, and solid-solid equilibria can be shown in the same diagram. This is not possible for a binary system. Phase diagram of pure solvent and solution. - ResearchGate Phase diagram of pure solvent and solution. Source publication Design and Evaluation of Multiple Effect Evaporator Systems According to the Steam Ejector Position Article Full-text available Jan...
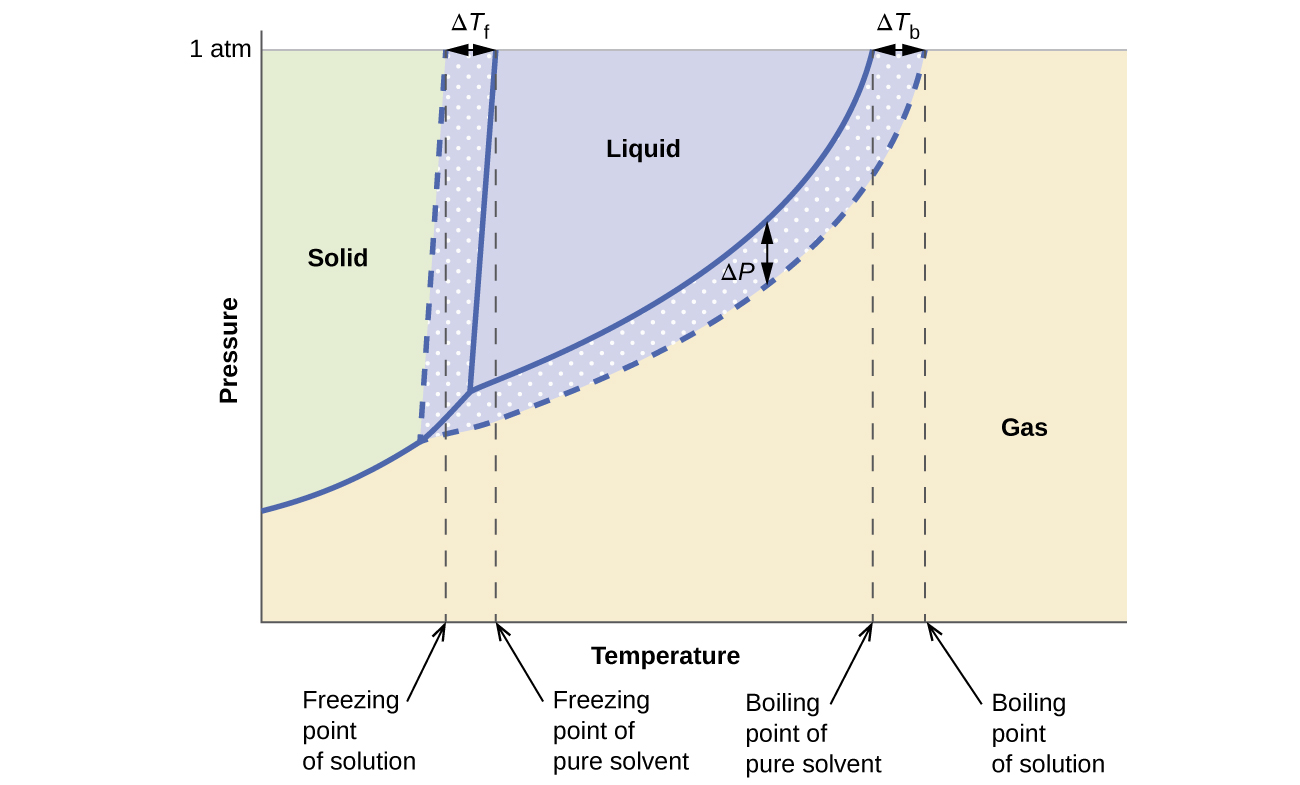
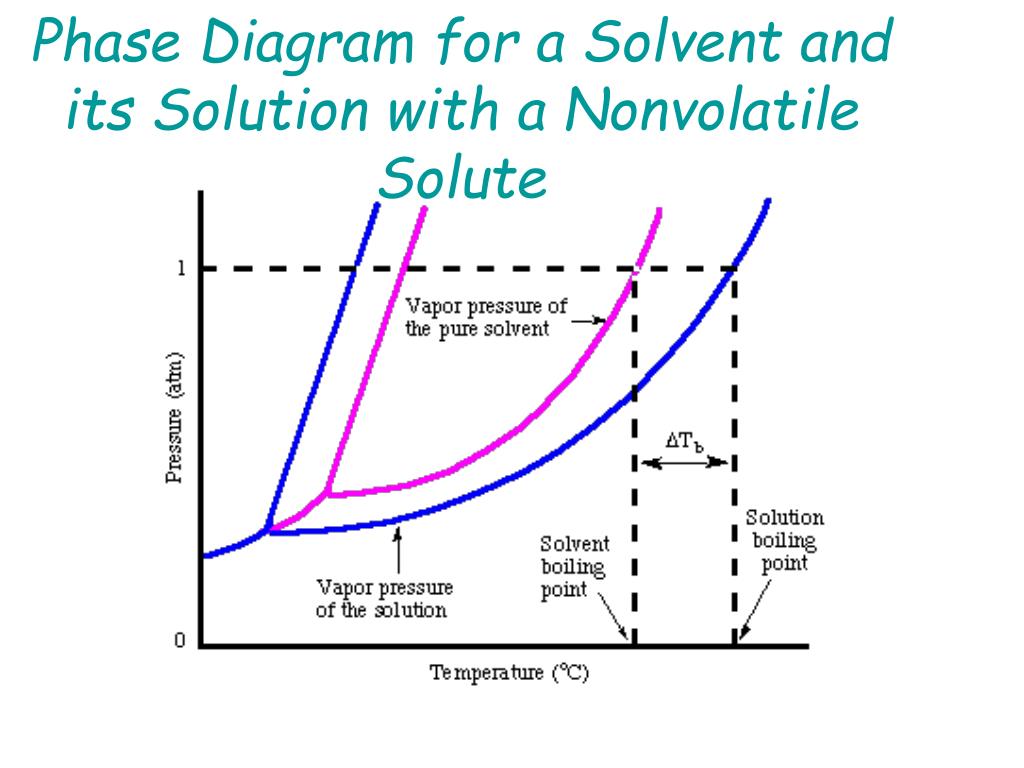
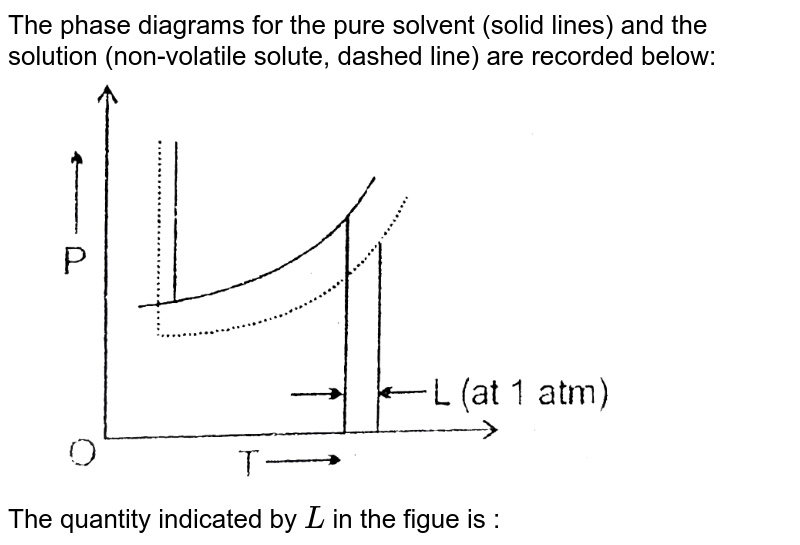
Phase diagram of pure solvent and solution. Mixed Solvent systems : Phasediagram In all the depicted phase diagrams, the markers represent experimental data while the solubility equilibrium lines were calculated with the Extended UNIQUAC model. Notice that the temperatures vary between the diagrams. In all the graphs below, the focus is on the lower part of the diagrams. The apex with pure K 2 SO 4 is therefore not shown. › ternary-phase-diagramTernary Phase Diagram - an overview | ScienceDirect Topics A ternary phase diagram shows possible phases and their equilibrium according to the composition of a mixture of three components at constant temperature and pressure. Figure 4.23 shows a schematic of a ternary phase diagram. Single-phase regions are areas that originate from the vertex of the triangle and that are not enclosed by black curves. pressbooks-dev.oer.hawaii.edu › phase-diagramsPhase Diagrams – Chemistry - University of Hawaiʻi A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase-transition temperatures ... The phase diagrams for the pure solvent (solid lines) and the solution ... asked Dec 24, 2021 in Chemistry by SupriyaRani (24.2k points) The phase diagrams for the pure solvent (solid lines) and the solution (non-volatile solute, dashed line) are recorded below. The quantity indicated by L in the figure is: A. Δp Δ p B. ΔT f Δ T f C. K bm K b m D. K f m K f m class-12 graphical-interpretation
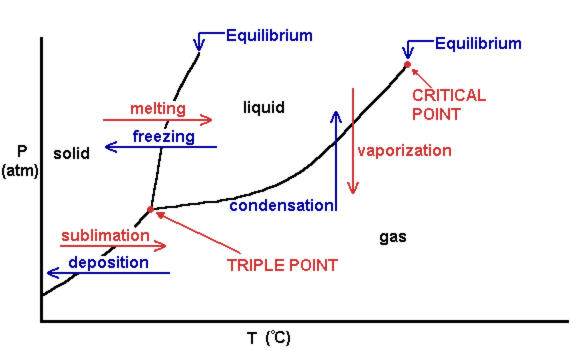
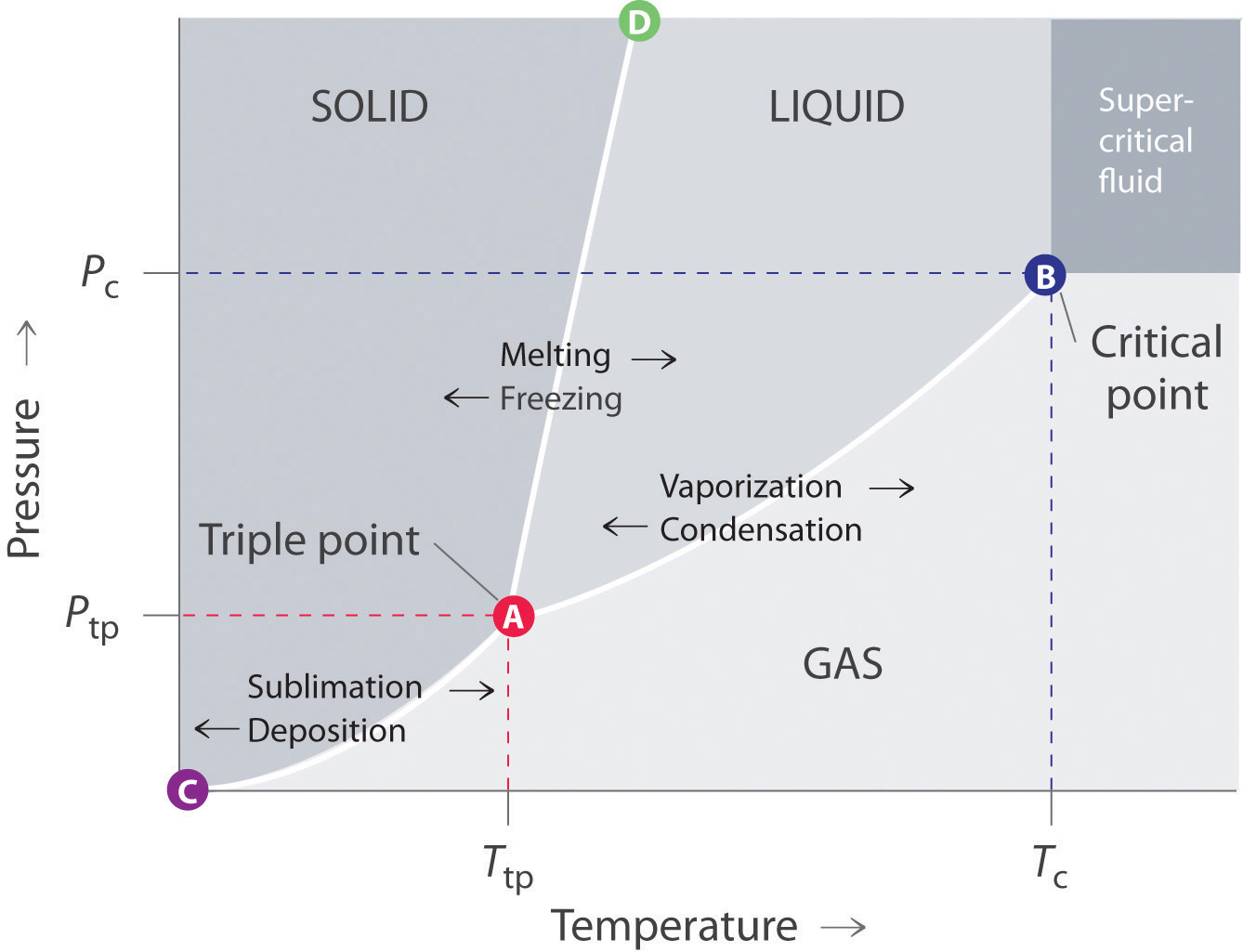
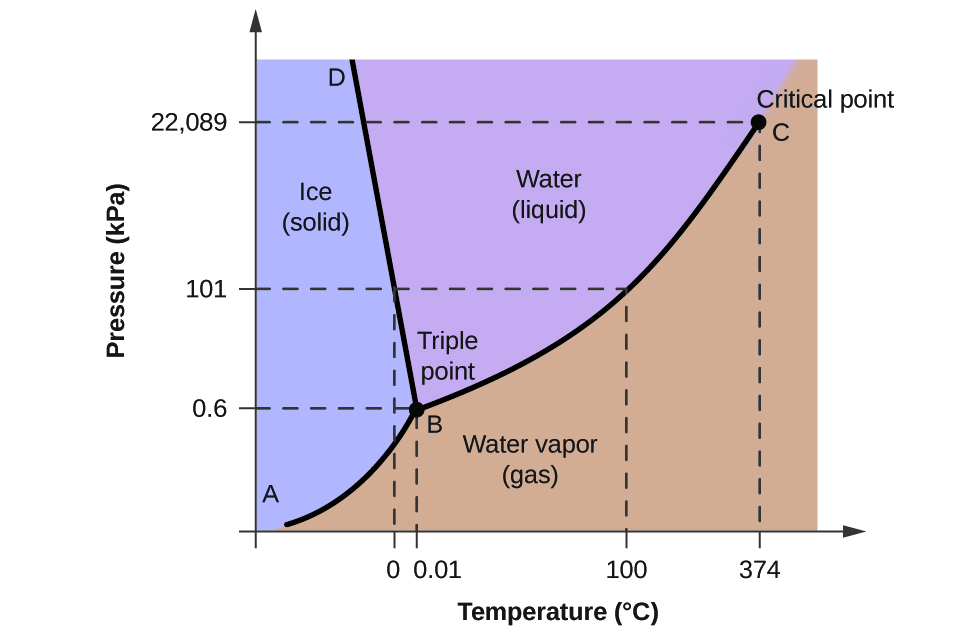
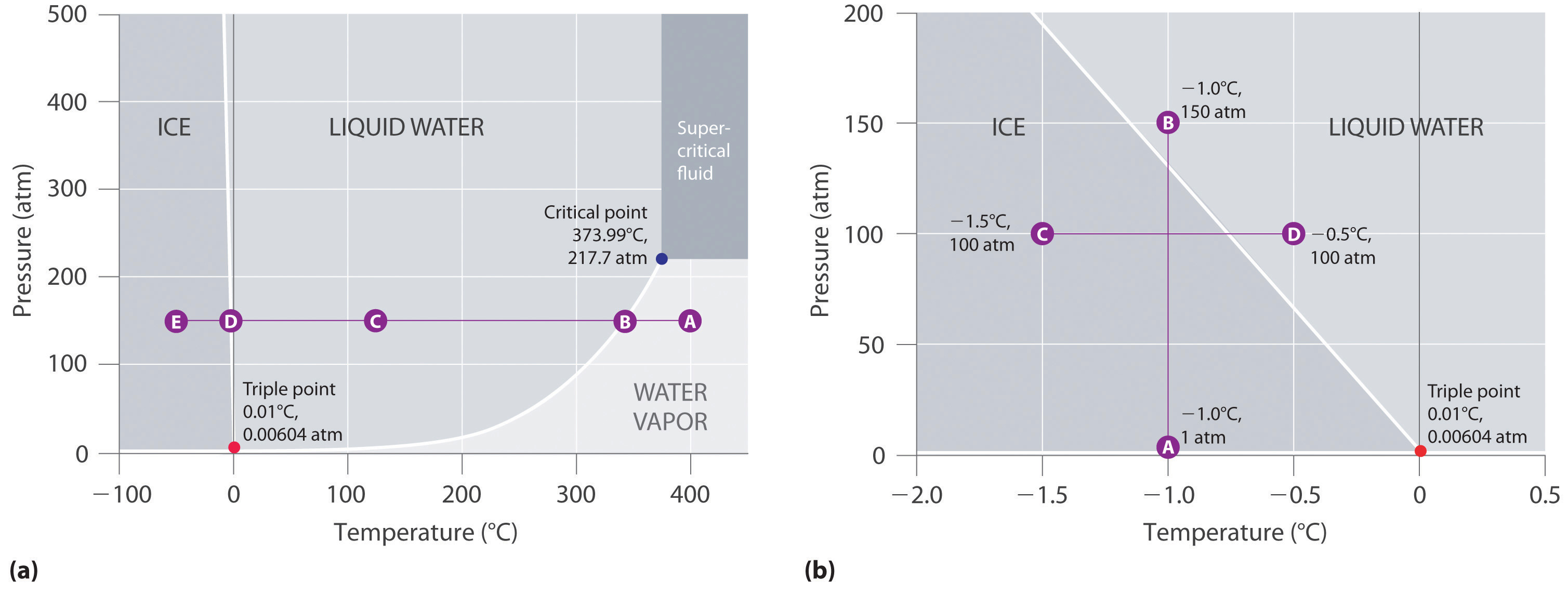
The phase diagram of a pure substance. - ResearchGate In a phase diagram of a pure substance ( Figure 5 ), the critical point is marked at the end of the gas-liquid equilibrium curve, and the gray area indicates the supercritical fluid region. At the ... 10.4 Phase Diagrams - Chemistry A typical phase diagram for a pure substance is shown in Figure 1. Figure 1. The physical state of a substance and its phase-transition temperatures are represented graphically in a phase diagram. To illustrate the utility of these plots, consider the phase diagram for water shown in Figure 2. Figure 2. phase diagrams of pure substances - chemguide A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. In the cases we'll be looking at on this page, the phases will simply be the solid, liquid or vapour (gas) states of a pure substance. This is the phase diagram for a typical pure substance. Answered: True or False 1.) The phase diagram of… | bartleby True or False 1.) The phase diagram of a pure solvent shows a lower freezing point compared to solutions 2.) The process of freezing only lowers the kinetic energy of solute particles in a solution 3.) Ionic solutes have higher van't hoff factor compared to non ionic solutes 4.) The formation of intermolecular forces of attraction requires ...
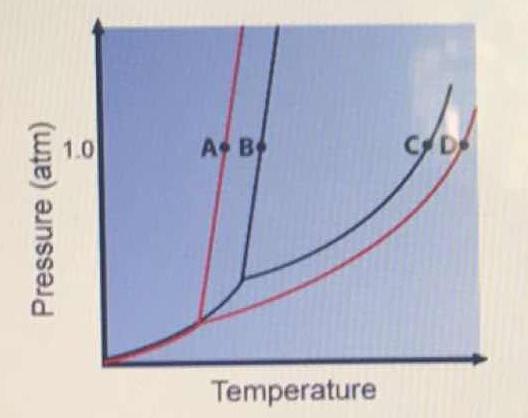
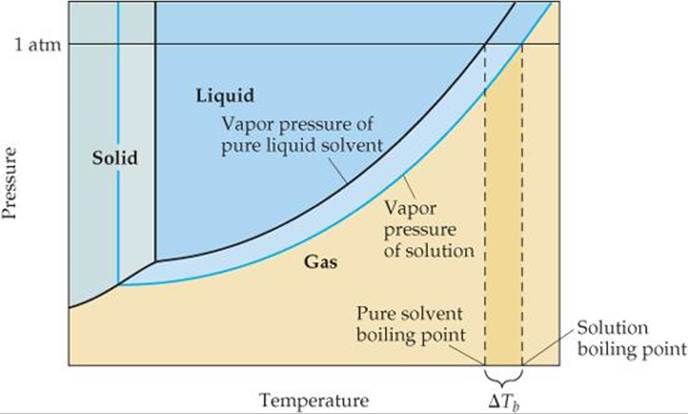
Phase Diagrams - General College Chemistry A typical phase diagram for a pure substance is shown in Figure 30.1. Figure 30.1 The physical state of a substance and its phase-transition temperatures are represented graphically in a phase diagram. To illustrate the utility of these plots, consider the phase diagram for water shown in Figure 30.2. Figure 30.2 en.wikipedia.org › wiki › Phase_(matter)Phase (matter) - Wikipedia Solubility is the maximum amount of a solute that can dissolve in a solvent before the solute ceases to dissolve and remains in a separate phase. A mixture can separate into more than two liquid phases and the concept of phase separation extends to solids, i.e., solids can form solid solutions or The figure shows two phase diagrams, one for a pure liquid (black line ... Similarly, the normal boiling point of the pure substance at constant pressure is indicated by C, and the raised normal boiling point of the solution with the pure liquid as the solvent (again, due to addition of nonvolatile solute to the pure liquid substance) is indicated by D, since T ↑ rightwards. ΔT b = T b − T * b = iKbm The phase diagram for solvent and solutions is shown in the figure ... The phase diagram for solvent and solutions is shown in the figure. What represents the normal boiling point of the solution? A A B B C C D D Hard Solution Verified by Toppr Correct option is D) The normal boiling point of the solution is that temperature at which vapour pressure of solution equals to 1 atm.
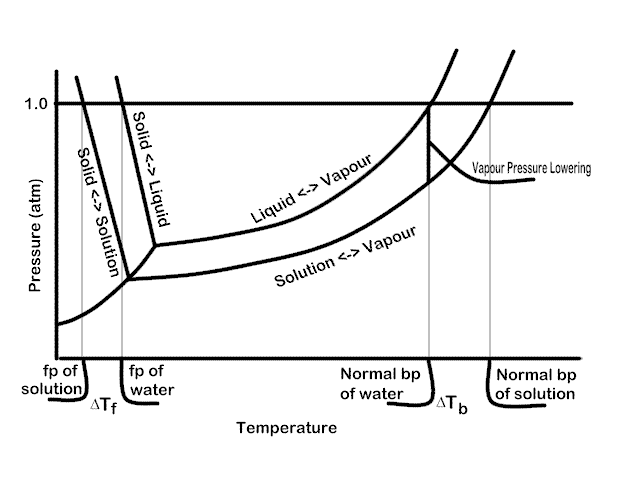
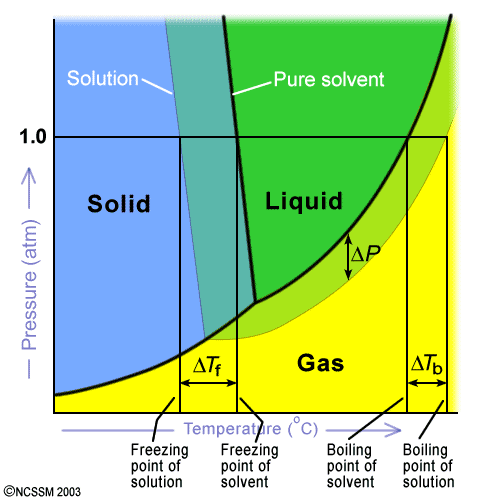
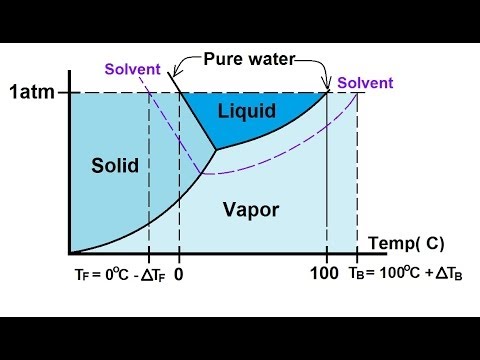
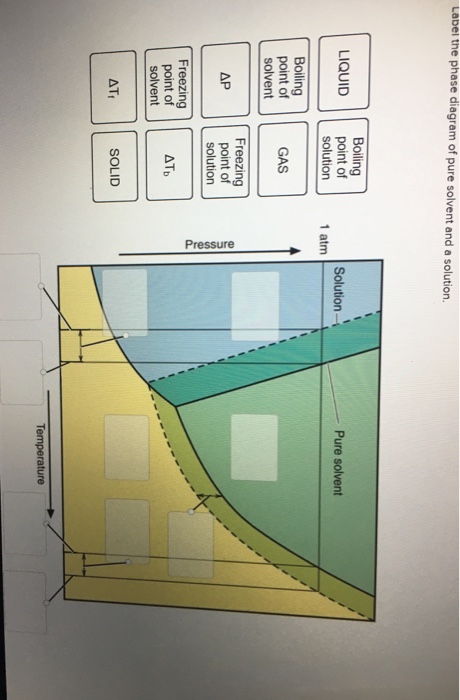
Solved The phase diagrams for a pure solvent and the solvent - Chegg Chemistry questions and answers. The phase diagrams for a pure solvent and the solvent in a solution are shown. Identify the normal freezing (fp_solv) and boiling (bp_solv) points for the pure solvent and the normal freezing (fp_soln) and boiling (bp_soln) points of the solution at 1 atm. Assume the solute is nonvolatile and that the solid that ...
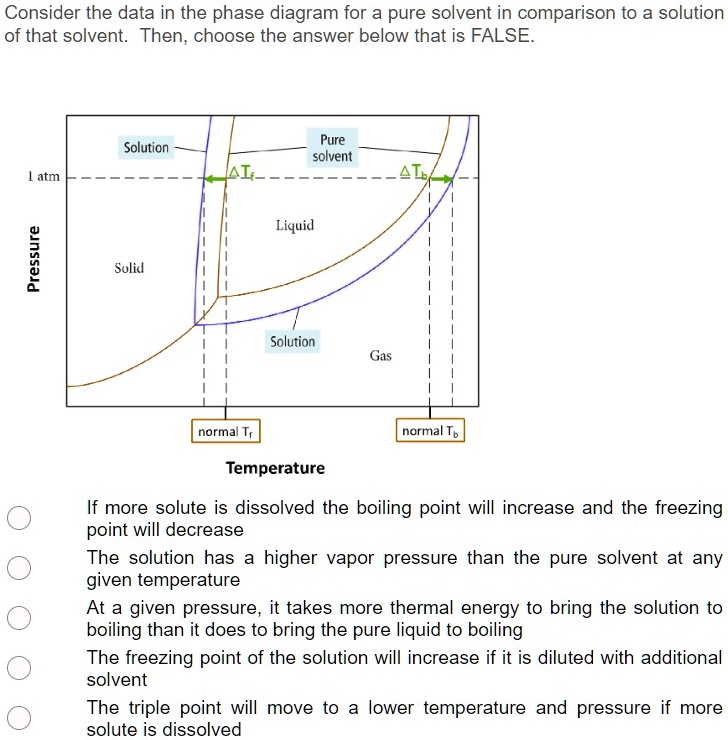
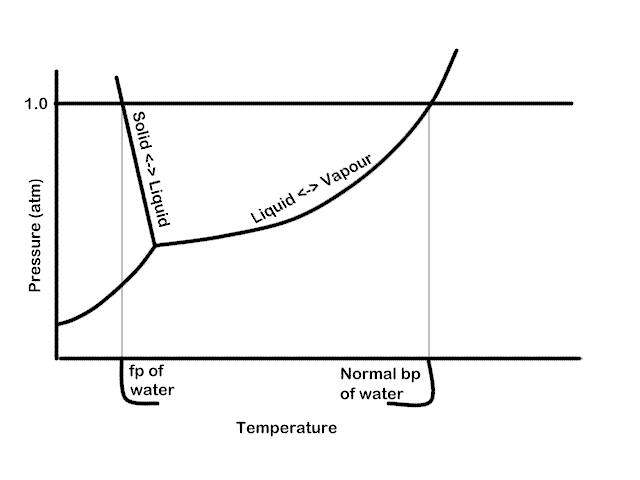
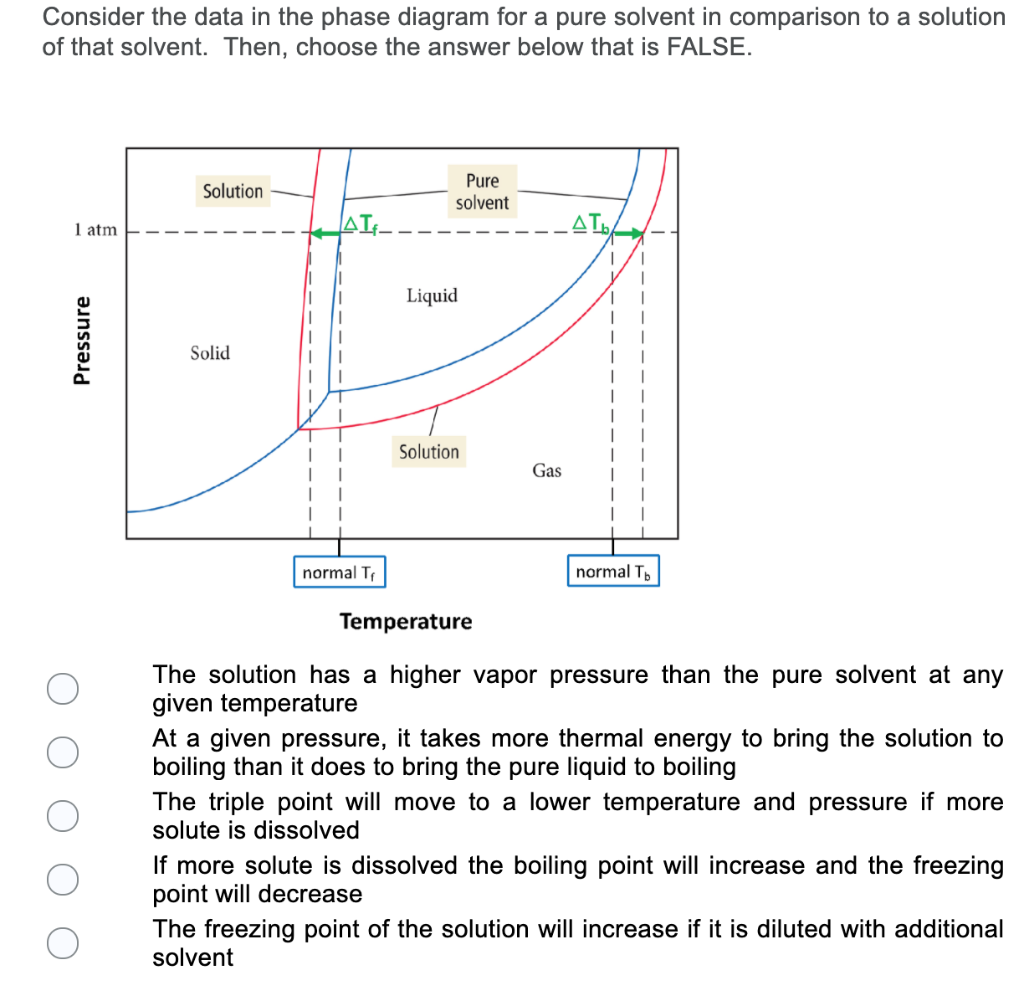
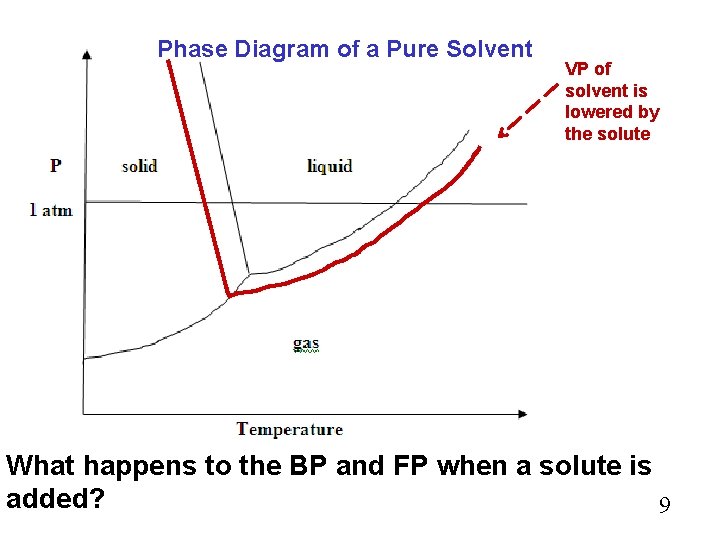
The important differences between the phase diagram of a pure solvent ... The important differences between the phase diagram of a pure solvent and that of the solution of that solvent are to be determined. Concept introduction: The phase diagram is a combination of liquid-gas, solid-liquid, and solid-gas curves and determines the effect of temperature and pressure on the phase of a substance.
Draw a phase diagram showing how the phase boundaries differ for a pure ... Problem 78 Medium Difficulty Draw a phase diagram showing how the phase boundaries differ for a pure solvent compared with a solution. Answer The phase boundaries are shown for both solvent and solution. The liquid gas boundary for a solution is at a higher temperature than that for the pure solvent. This indicates the elevation in boiling point.
solid-liquid phase diagrams: salt solution - chemguide We'll take a solution containing 100 g of potassium nitrate and 100 g of water. Now let the solution cool. At all temperatures above that marked on the graph (about 57°C), 100 g of water will dissolve more than 100 g of potassium nitrate. All the potassium nitrate will stay in solution. At 57°C, you hit the solubility curve.
Liquid and Solid Solution phase changes - First Year General Chemistry Liquid solution and pure A solid. IV. Liquid solution and pure AB 2 solid. V. Liquid solution and pure B solid. Two component Na/K phase diagram showing incongruent melting compound Na2K and amalgams (solid phase solutions) of Na in K or of K in Na. This final phase diagram shows a two component system made up of Na and K metals in different ...
› 31613237 › _Easterling_Kenneth_E[Easterling, Kenneth E.; Porter, Phase Transformations in ... Easterling, K. E. Porter, David A. Sherif, Mohamed Y Phase transformations in metals and alloys CRC Press (2009) by matheus andrade Download Free PDF Download PDF Download Free PDF View PDF
PDF phase diagram solvent solution - Just Only phase diagram solvent solution Effect of Solute on Phase Diagram of Water SOLIDLIQUID GAS Pure solvent Freezing point of solution Freezing point of water Boiling point of water Boiling point of solution 1 atm Pressure ΔTfΔTb ΔP Temperature
A phase diagram showing how the phase boundaries differ for a pure ... A phase diagram showing how the phase boundaries differ for a pure solvent compared with a solution has to be drawn. Concept introduction: Phase diagramis a graphical picture of the physical states of a substance under different conditions of temperature and pressure.
Phase diagrams for ternary salt solutions : Phasediagram To the left in the diagram, at zero Na 2 O content, the solution consists of water and pure phosphoric acid, H 3 PO 4. The melting point of pure H 3 PO 4 is approximately 40°C. The solid-liquid equilibrium line therefore does not start in the 60°C phase diagram until there is enough sodium content for NaH 2 PO 4 ·H 3 PO 4 to form.
PDF Phase Diagrams, Solid Solutions, Phase Transformations Isomorphous Phase Diagrams Phase diagram Indicate phases as a function of Temp., Comp. and Pressure (under equilibrium condition) Binary phase diagram A phase diagram for a system with two components. Isomorphous phase diagram A phase diagram in which the components display unlimited solid solubility. Chapter 10: Solid Solutions and Phase ...
› resources › glossaryGlossary of Stainless Steel Terms - Unified Alloys Phase A physically homogeneous and distincy portion of a material system. Phase Diagram Synonymous with constitutional diagram. Phosphor Bronze Copper base alloys, with 3.5 to 10% of tin, to which has been added in the molten state phosphorus in varying amounts of less than 1% for deoxidizing and strengthening purposes. Because of excellent ...
PDF Chapter 9: Phase Diagrams Phase Equilibria: Solubility Limit 65 Sucrose/Water Phase Diagram Pure Sugar emperature (°C) 0 20 40 60 80 100 C o =Composition (wt% sugar) L (liquid solution i.e., syrup) Solubility Limit L (liquid) + S (solid 20 sugar) 4 0 6 0 8 0 10 0 Pure ater Question: What is the solubility limit at 20C? Answer: 65wt% sugar. If Co < 65wt% sugar: syrup If ...
en.wikipedia.org › wiki › Aqueous_solutionAqueous solution - Wikipedia For example, a solution of table salt, or sodium chloride (NaCl), in water would be represented as Na + (aq) + Cl − (aq). The word aqueous (which comes from aqua) means pertaining to, related to, similar to, or dissolved in, water. As water is an excellent solvent and is also naturally abundant, it is a ubiquitous solvent in chemistry. Since ...
Solved The phase diagrams for a pure solvent and the solvent - Chegg Chemistry questions and answers. The phase diagrams for a pure solvent and the solvent in a solution are shown. Identify the normal freezing (fpsoly) and boiling (bpsolv) points for the pure solvent and the normal freezing (fpsoln) and boiling (bpsoln) points of the solution at 1 atm. Assume the solute is nonvolatile and that the solid that ...
Answered: The phase diagrams for a pure solvent… | bartleby identify the normal freezing (fpsolv) and boiling (bpsolv) points for the pure solvent and the normal freezing (fpsoln) and boiling (bpgoln) points of the solution at 1 atm. assume the solute is nonvolatile and that the solid that freezes from solution is pure solvent. 1 atm liquid solid answer bank fpsolv bpsolv fpsoln bpsoln gas temperature …
Microsoft Excel Solver-assisted Composition Determination to Improve ... Here, as shown in Figure 4, the phase diagrams of three binary systems have been constructed, including ethanol−cyclohexane, ethanol−ethyl acetate, and ethanol−isopropanol, selected for their mild operation temperature, green and less corrosive solvents for safe experimentation. The phase diagrams constructed from the compositions solved ...
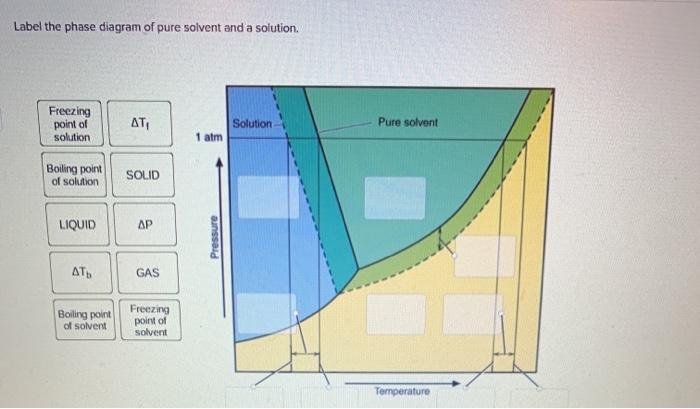
Question : Label the phase diagram of pure solvent and a solution. - Chegg Expert Answer. 100% (137 ratings) This is …. View the full answer. Transcribed image text: Label the phase diagram of pure solvent and a solution.
Phase diagram of pure solvent and solution. - ResearchGate Phase diagram of pure solvent and solution. Source publication Design and Evaluation of Multiple Effect Evaporator Systems According to the Steam Ejector Position Article Full-text available Jan...
Process design and phase diagrams : Phasediagram Phase diagrams for pure compounds are visual tools to display the properties of pure compounds forming various phases at different temperatures and pressures. In a phase diagram for a pure component, solid-liquid, vapor-liquid, solid-vapor, and solid-solid equilibria can be shown in the same diagram. This is not possible for a binary system.
› topics › chemistryGreen Solvent - an overview | ScienceDirect Topics 4.1.1 NaOH/urea aqueous solution. As a novel green solvent, NaOH/urea aqueous solution was first used for the rapid dissolution of cellulose at low temperature by Zhang et al. [1]. Due to its excellent performance for the dissolution of biomacromolecules, Hu et al. first used NaOH/urea aqueous solution for the dissolution of chitin [2].










Post a Comment for "38 phase diagram of pure solvent and solution"